![SOLVED: What is the [H3O+] and [OH-] of a vinegar solution whose pH is 2.60? [H3Ot] -= 2.51x 10-3M [OH:] = 4.76x 10-10 , M [H3Ot]= 251x 10-3 M [OH] = 3.98x SOLVED: What is the [H3O+] and [OH-] of a vinegar solution whose pH is 2.60? [H3Ot] -= 2.51x 10-3M [OH:] = 4.76x 10-10 , M [H3Ot]= 251x 10-3 M [OH] = 3.98x](https://cdn.numerade.com/ask_images/6dfe549d4995435d873f5dcfa09e91b7.jpg)
SOLVED: What is the [H3O+] and [OH-] of a vinegar solution whose pH is 2.60? [H3Ot] -= 2.51x 10-3M [OH:] = 4.76x 10-10 , M [H3Ot]= 251x 10-3 M [OH] = 3.98x
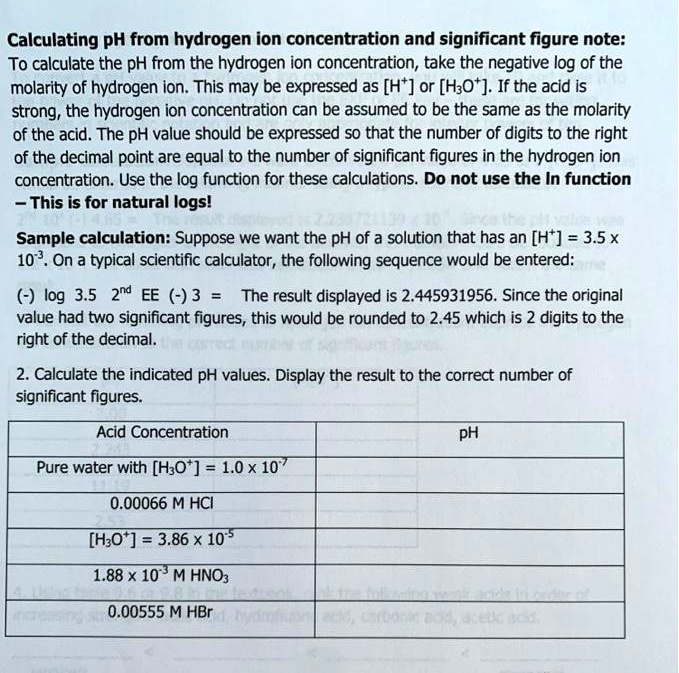
SOLVED: Calculating pH from hydrogen ion concentration and significant figure note: To calculate the pH from the hydrogen ion concentration; take the negative log of the molarity of hydrogen ion. This may
![SOLVED: What are the concentrations of H3O and OH– in oranges that have a pH of 3.98? [H3O+]= M [OH-]= M SOLVED: What are the concentrations of H3O and OH– in oranges that have a pH of 3.98? [H3O+]= M [OH-]= M](https://cdn.numerade.com/ask_previews/e9f7adf9-a742-4ec8-bdea-5a0f18192bbf_large.jpg)
SOLVED: What are the concentrations of H3O and OH– in oranges that have a pH of 3.98? [H3O+]= M [OH-]= M
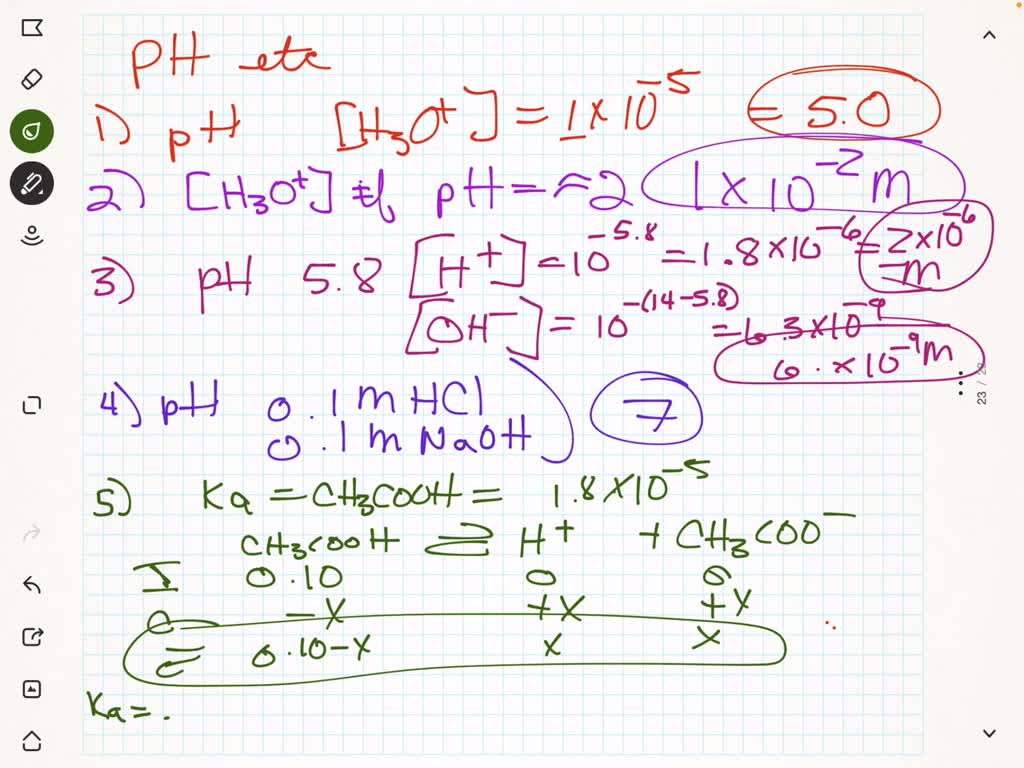
SOLVED: 1. What is the pH of a urine sample that has an H3O+ concentration of 1 x 10-5 M? Classify the solution as acidic, basic or neutral. 2. What is the

Calculer le pH d'un acide fort & d'une base forte avec les approximations - E-Learning Chimie - YouTube

Vidéo de question : Calculer la concentration en ions hydronium d'une solution à partir de son pOH | Nagwa