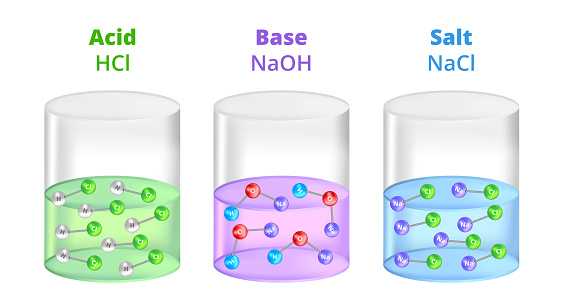
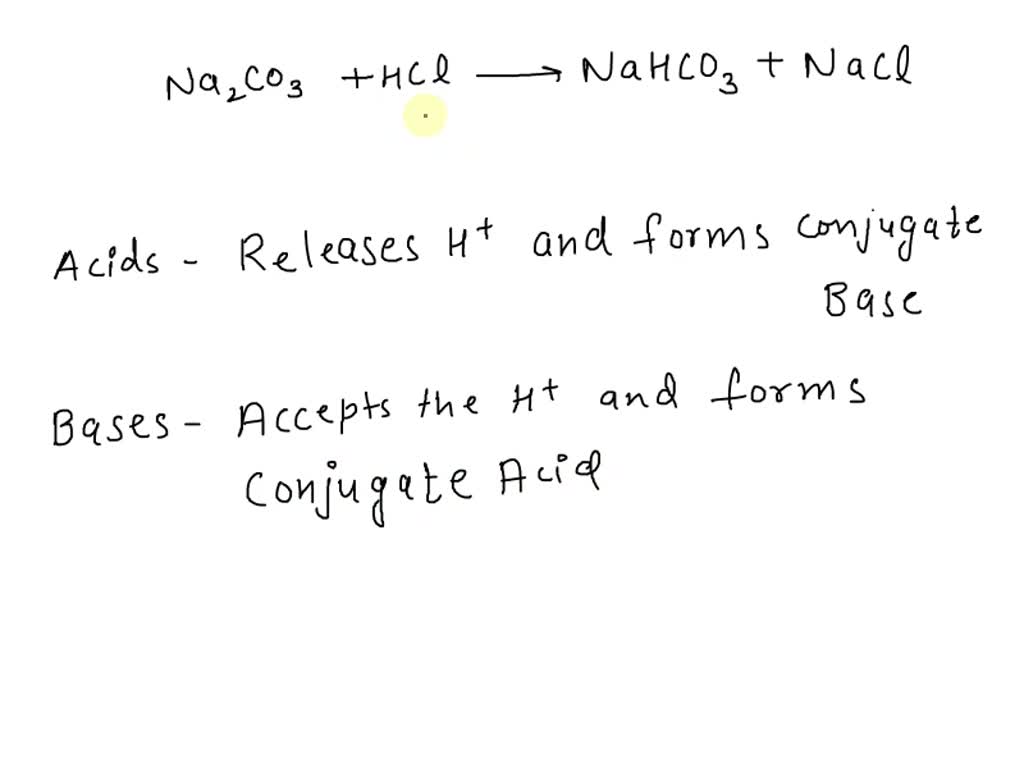
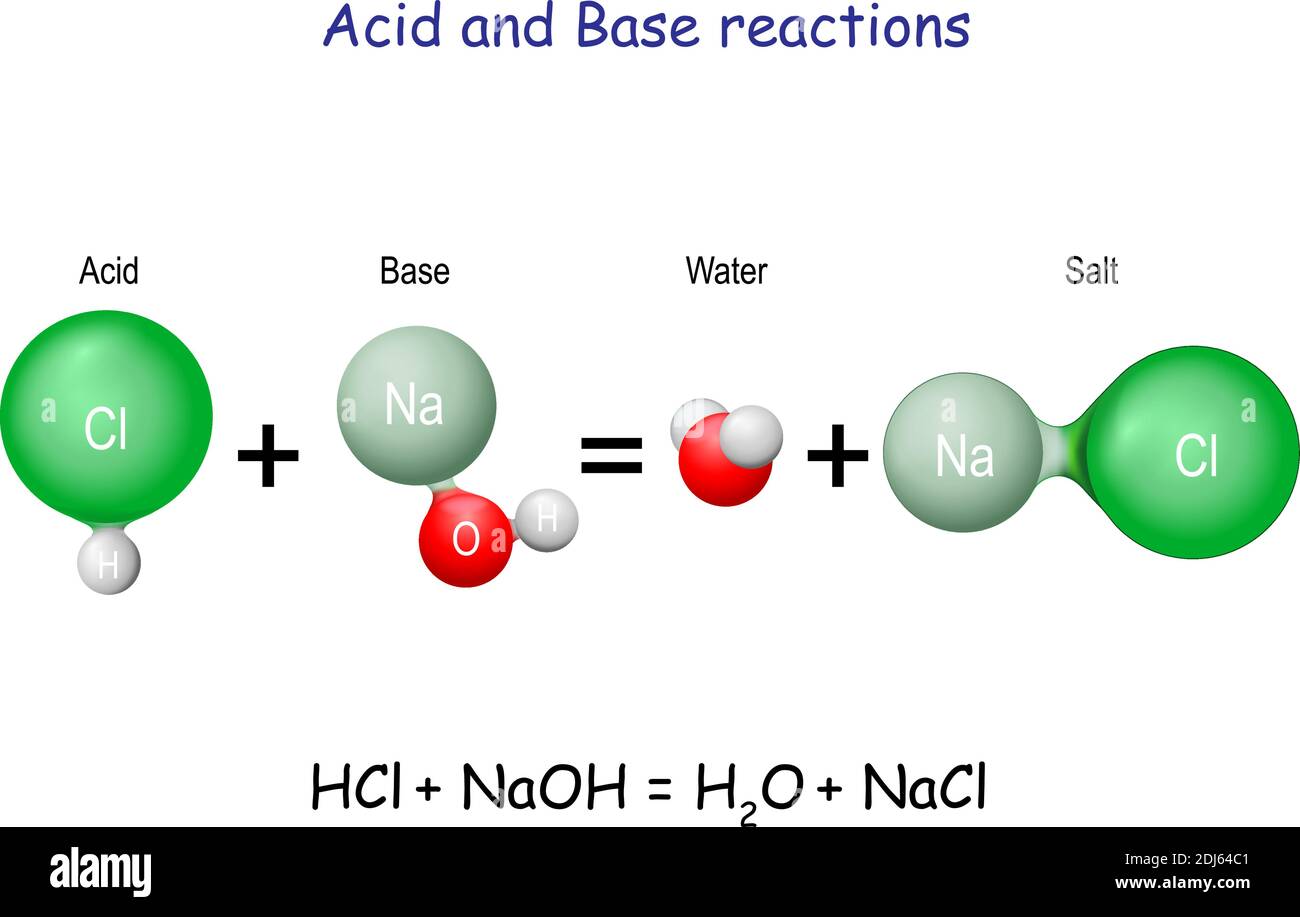
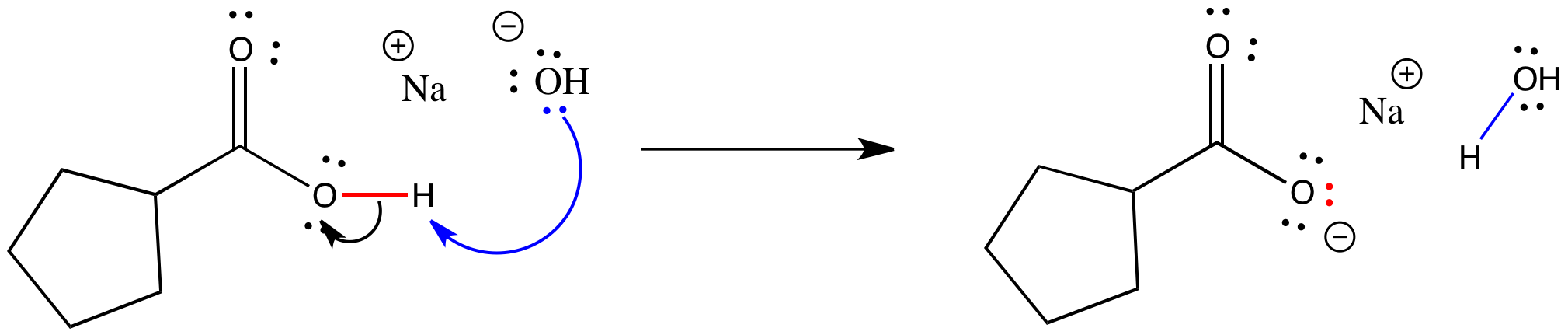
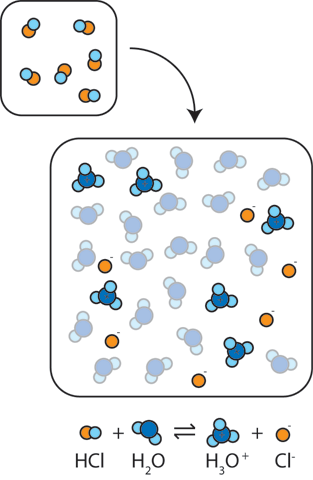
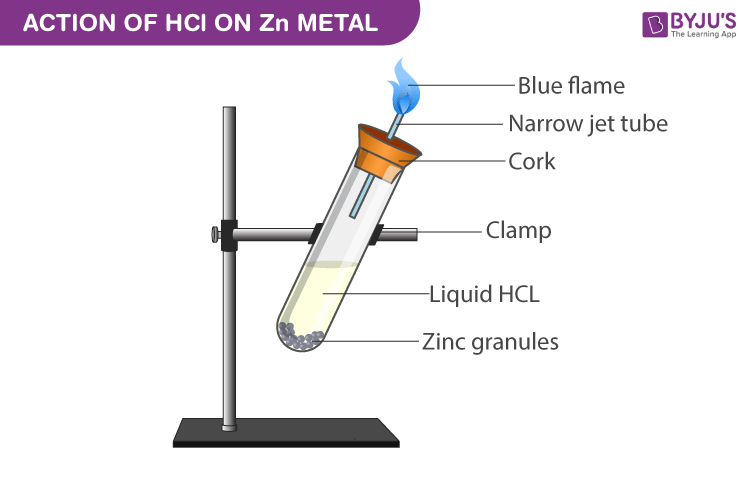
Acid – base reaction. chemical reaction neutralization the acid and base properties, producing a salt and water. used to determine pH. Bronsted – Lowry theory. molecules of HCl, NaOH, H2O, and NaCl,

Write the neutralization reaction for the following acid and base: HCl_{(aq)} and KOH_{(aq)}. | Homework.Study.com
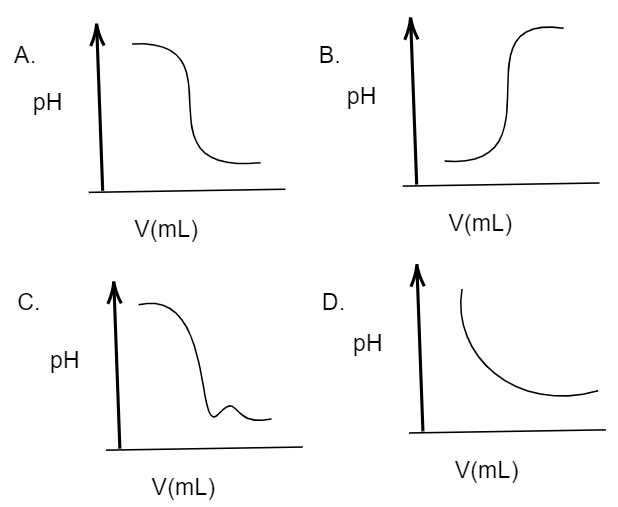
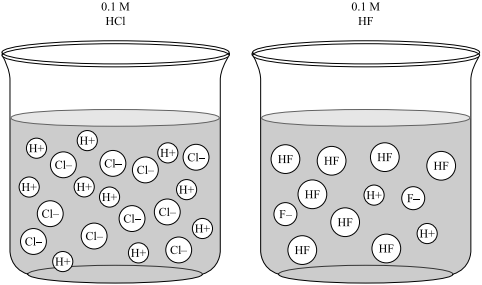
In an acid-base titration, $0.1M$ $HCl$ solution was added to the $NaOH$ solution of unknown strength. Which of the following correctly shows the change of pH of the titration mixture in this

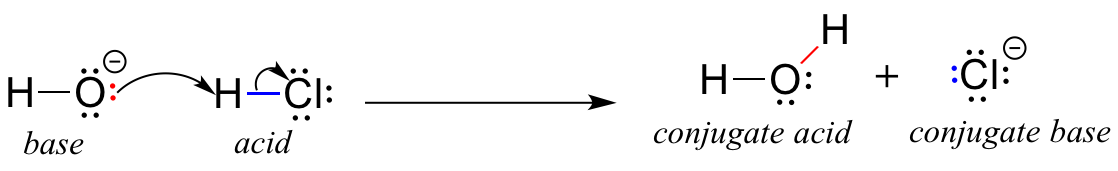
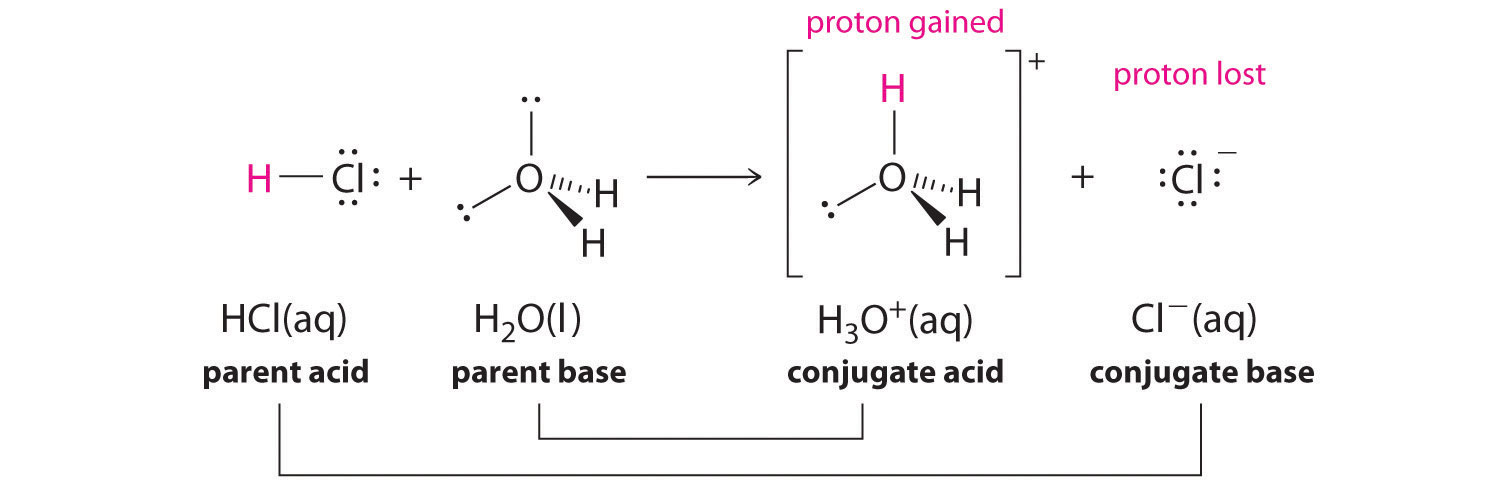
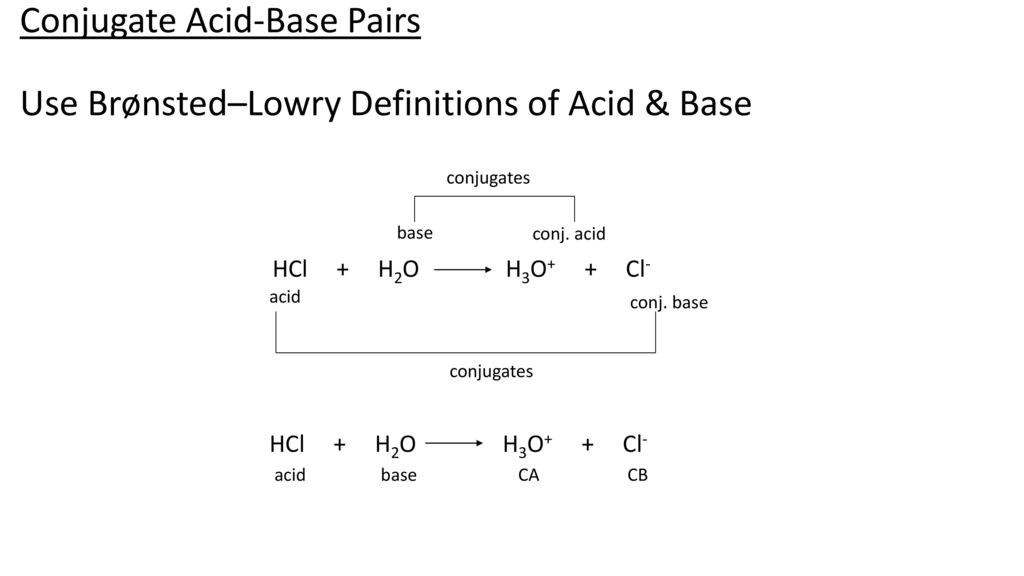
The ionization of hydrochloric acid in water is given below: HCl(aq) + H2O(l) H3O^+(aq) + Cl^-(aq) Lable two conjugate acid - base pairs in this ionization.