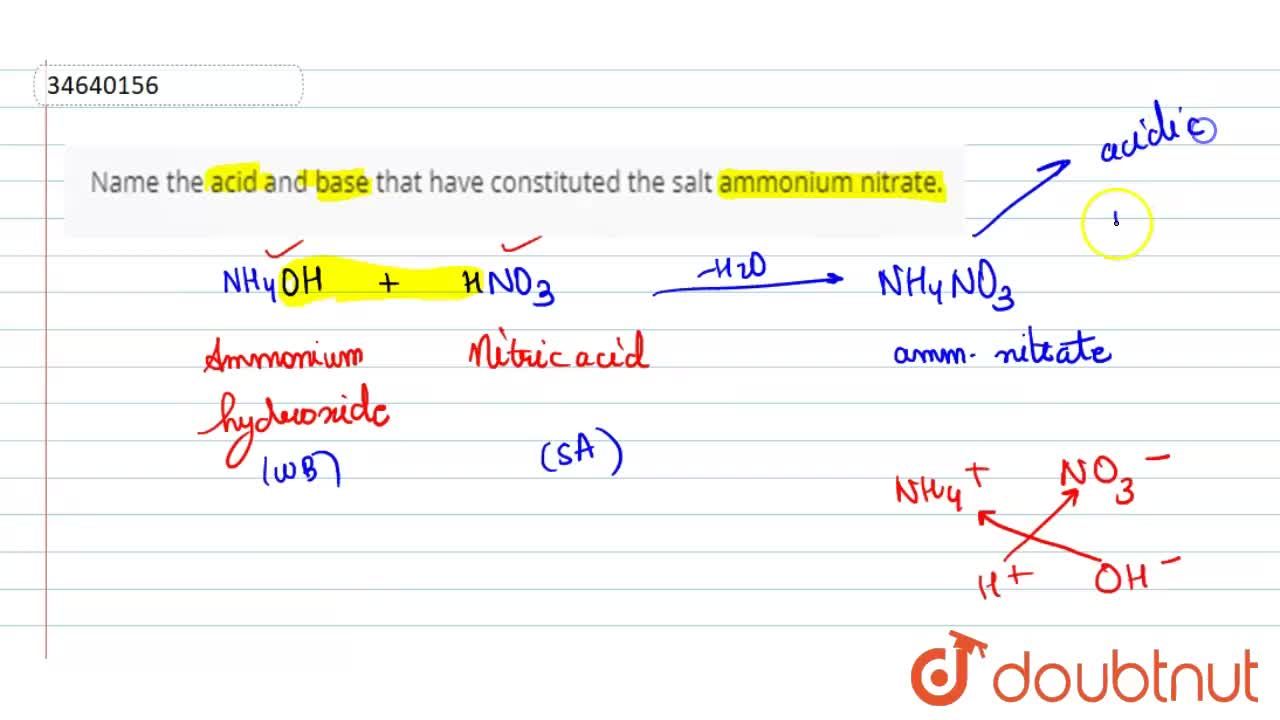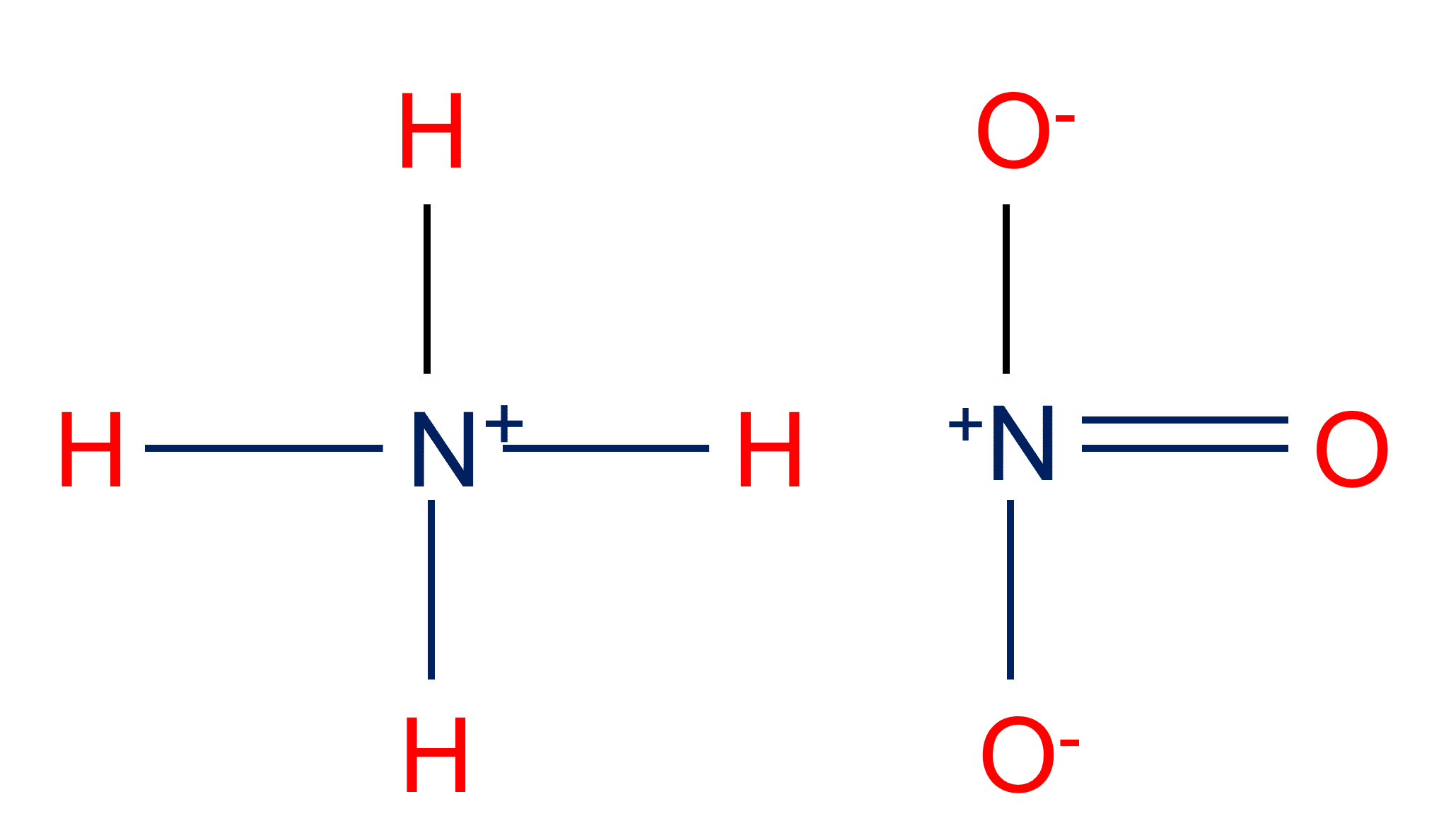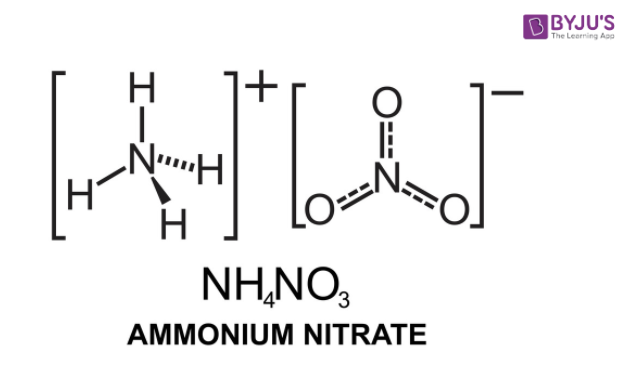
Ammonium Nitrate (NH<sub>4</sub>NO<sub>3</sub>) - Structure, Preparation, Physical and Chemical Properties, Uses with FAQs of Ammonium Nitrate

Ammonium Nitrate (NH<sub>4</sub>NO<sub>3</sub>) - Structure, Preparation, Physical and Chemical Properties, Uses with FAQs of Ammonium Nitrate

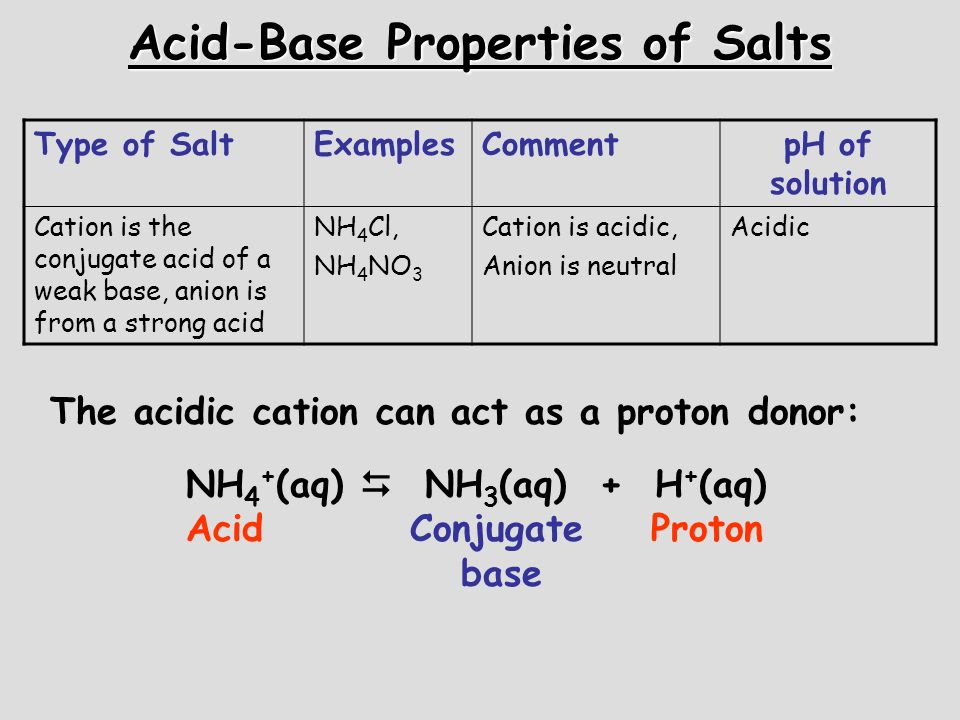
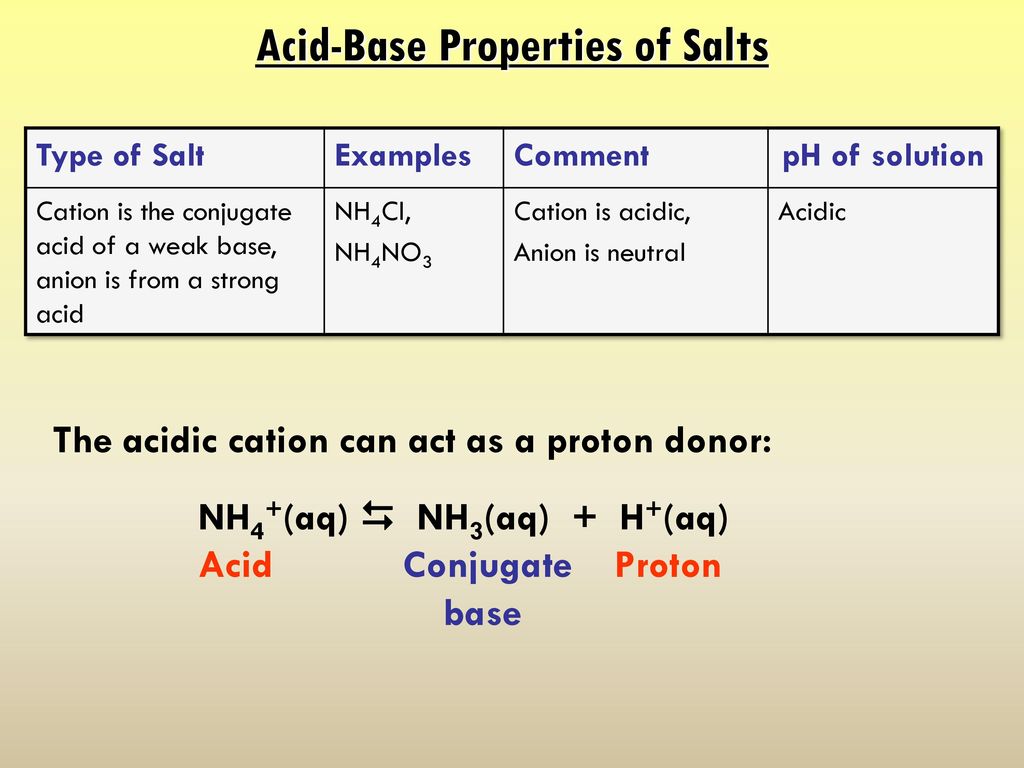
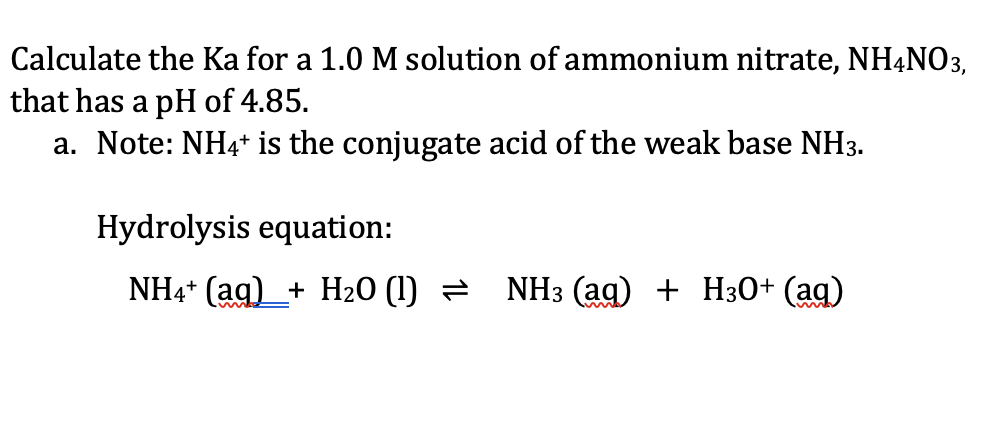
OneClass: Ammonium nitrate, NH4NO3, is a salt formed from the neutralization of the weak base ammonia...
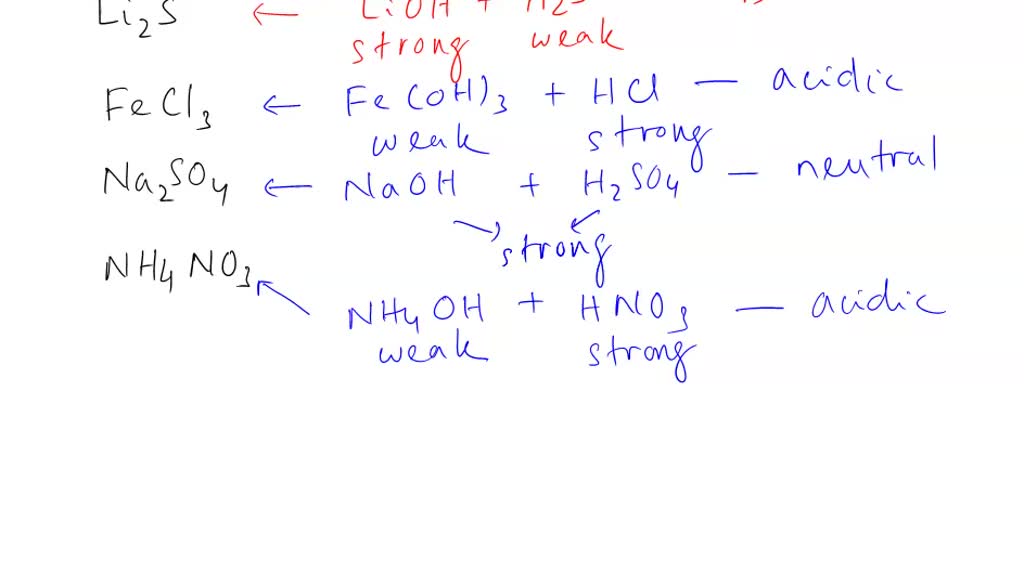
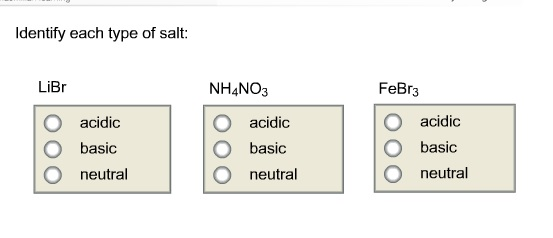
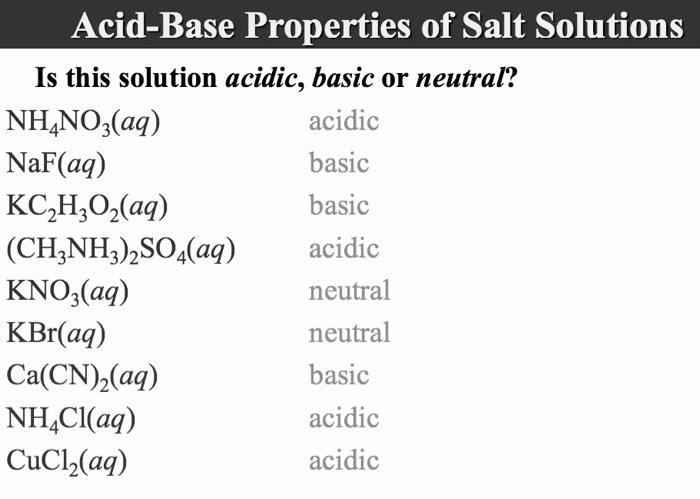
SOLVED: What is the equation for hydrolysis for anion and cation. Determine whether salt is acidic, basic or neutral. 1.) K3PO3 2.) Li2S 3.) FeCl3 4.) Na2SO4 5.) NH4NO3

Predict if the solutions of the following salts are neutral, acidic or basic. NaCl, KBr, NaCN, NH4NO3,NaNO2 and KF

SOLVED: Write an ionic equations for the hydrolysis reactions of NH4NO3 and Na2SO3. Predict the pH of the solution equal 7, greater than 7, less than7).